Oh, hello there! Today, I wanted to talk to you about CAR T-cell therapy – have you heard of it? If not, don't worry – I'm here to break it down for you.
What is CAR T-cell therapy?
Simply put, CAR T-cell therapy is a type of immunotherapy that uses a patient's own immune cells to fight cancer. The therapy involves collecting a patient's T-cells (a type of white blood cell that fights infection) and genetically modifying them in a lab to produce what are known as chimeric antigen receptors (CARs). These CARs enable the T-cells to recognize and attack cancer cells when they are reintroduced into the patient's body.
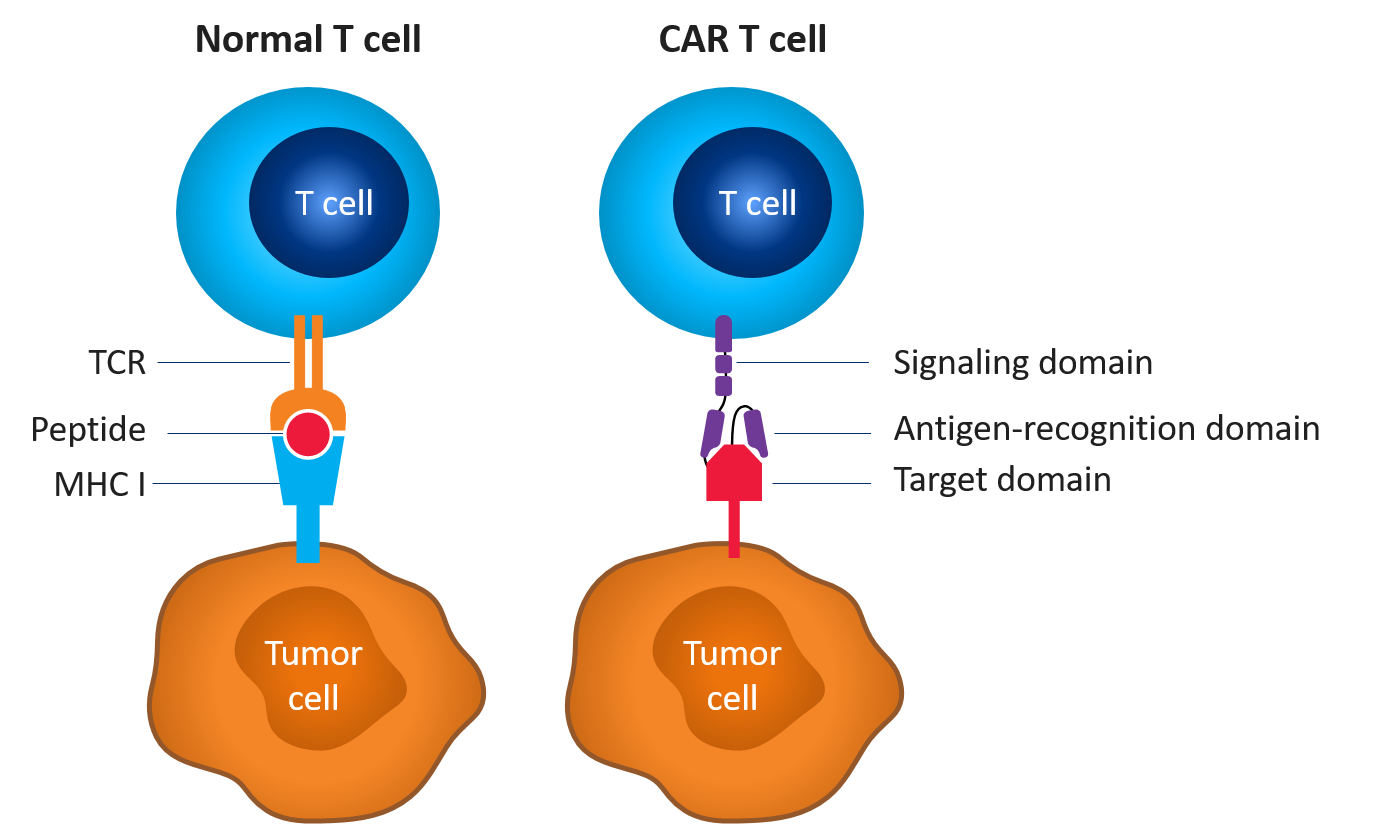
Now, at this point you might be thinking – "wait, so you're telling me that scientists can just reprogram cells to fight cancer? That sounds too good to be true!" And I get it – it does sound like something out of a sci-fi movie. But the truth is, CAR T-cell therapy has actually been used in clinical trials since the early 2000s, and has shown some really promising results.
How is CAR T-cell therapy used?
CAR T-cell therapy is currently approved for use in two types of cancer – acute lymphoblastic leukemia (ALL) and advanced diffuse large B-cell lymphoma (DLBCL). For patients with these types of cancer, CAR T-cell therapy is typically used as a treatment of last resort – meaning, it is only used when other treatments have failed.
One thing to note is that CAR T-cell therapy is a highly personalized treatment – meaning, it is tailored to each individual patient. This is because the therapy involves collecting a patient's own immune cells, modifying them in a lab, and then reintroducing them back into the patient's body. So while the overall process of CAR T-cell therapy is the same for every patient, the specific T-cells used and the genetic modifications made to those T-cells will vary from patient to patient.
What are the potential side effects of CAR T-cell therapy?
Like any medical treatment, CAR T-cell therapy comes with potential risks and side effects. Some of the most common side effects of CAR T-cell therapy include:
- Fever and flu-like symptoms
- Low blood pressure
- Neurological side effects, such as confusion and seizures
- Cytokine release syndrome (CRS), which is a type of immune response that can cause fever, fatigue, and other symptoms
It's worth noting that while these side effects can be serious, they are typically manageable with medication and close monitoring by a medical team. And, of course, the potential benefits of CAR T-cell therapy (such as extended remission times) should always be weighed against the risks before deciding whether or not to pursue the treatment.
What does the future of CAR T-cell therapy look like?
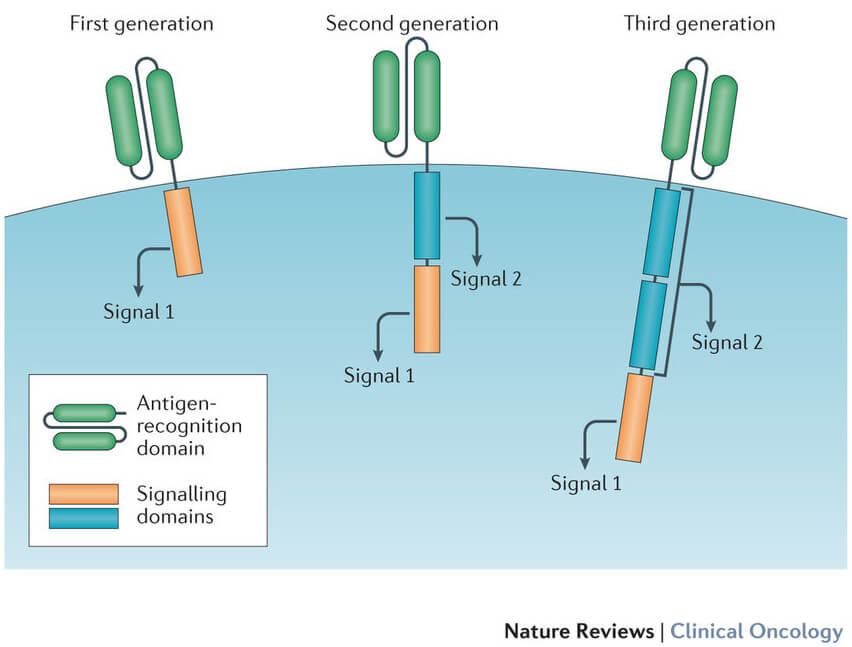
While CAR T-cell therapy is still a relatively new treatment, there is a lot of excitement around its potential – both in terms of treating new types of cancer, and improving the therapy itself. For example, researchers are currently exploring the use of CAR T-cells in other types of cancer, such as multiple myeloma and solid tumors. Additionally, there is ongoing research into ways to improve the efficacy of CAR T-cell therapy while minimizing side effects – for example, by using "off the shelf" CAR T-cell products that can be used across multiple patients.

Ultimately, CAR T-cell therapy represents an exciting new frontier in cancer treatment – one that has brought hope to many patients who had previously been told there were no other options. If you or a loved one is considering CAR T-cell therapy, be sure to talk to your doctor about whether it might be a good option for your specific situation.
Conclusion
So there you have it – a brief introduction to CAR T-cell therapy. While there is still much we don't know about this treatment, the early results are promising, and it has already helped many cancer patients who had previously been told there was no hope. Of course, like any medical treatment, CAR T-cell therapy comes with its own set of risks and potential side effects – but for many patients, the benefits may outweigh the risks. As always, be sure to talk to your doctor if you are considering CAR T-cell therapy for yourself or a loved one.
Thank you for taking the time to read this post – I hope you found it informative and helpful. If you have any questions or comments about CAR T-cell therapy, please feel free to leave them below!
References:
- National Cancer Institute: CAR T-cell therapy
- American Cancer Society: Cancer immunotherapy
- Food and Drug Administration: Chimeric antigen receptor T-cell therapy (CAR T cells)
If you are searching about Jimmy Fund - Dana-Farber and Brigham and Women's researchers laud FDA you've visit to the right place. We have 8 Images about Jimmy Fund - Dana-Farber and Brigham and Women's researchers laud FDA like New CAR T-cell therapy extends remission in heavily relapsed multiple, Managing the Side Effects in a CAR T-cell Therapy Study - Medelis and also Chimeric Antigen Receptor (CAR) T-Cell Therapy | AllCells®. Here you go:
Jimmy Fund - Dana-Farber And Brigham And Women's Researchers Laud FDA
car cell dana farber therapy lymphoma releases fda hodgkin non brigham diagram fund jimmy laud approval researchers jacobson caron cancer
Chimeric Antigen Receptor (CAR) T-Cell Therapy | AllCells®
 allcells.com
allcells.com antigen chimeric buffy
New CAR T-cell Therapy Extends Remission In Heavily Relapsed Multiple
 www.utsouthwestern.edu
www.utsouthwestern.edu myeloma immunotherapy remission relapsed extends heavily eurekalert biolabs
A Brief Introduction To CAR T-cell Therapy > Premier Research
 premier-research.com
premier-research.com cell car therapy vs normal test brief introduction premier research md gardner locke optimizing driving credit december cars
CAR T-Cell Therapy - Johns Hopkins Medicine
 clinicalconnection.hopkinsmedicine.org
clinicalconnection.hopkinsmedicine.org cell car therapy medicine video
CAR T-Cell Therapy For Treatment Of Leukemia -CancerFax
car therapy cell treatment leukemia chimeric antigen india receptors
Car T Cell Therapy Conference 2023
 copddiagnosis.web.fc2.com
copddiagnosis.web.fc2.com Managing The Side Effects In A CAR T-cell Therapy Study - Medelis
 medelis.com
medelis.com cell car therapy cells cancer immune engineering side second signaling effects study research generations receptor therapies improve stimulatory lymphoma treatment
Car t cell therapy conference 2023. Car therapy cell treatment leukemia chimeric antigen india receptors. Car cell dana farber therapy lymphoma releases fda hodgkin non brigham diagram fund jimmy laud approval researchers jacobson caron cancer
Post a Comment
Post a Comment